Gregory F. Payne
Affiliate Research Professor
Fischell Institute Fellow
Brain and Behavior Institute
Robert E. Fischell Institute for Biomedical Devices
A. James Clark School of Engineering
5118 A. James Clark Hall
gpayne@umd.edu
(301) 405-8389
Homepage
EDUCATION
Ph.D., The University of Michigan, 1984
HONORS AND AWARDS
- Guest Professor, Wuhan University, China
Biology is expert in creating functional nanoscale components (e.g., proteins) and assembling them over a hierarchy of length scales to create functional structures (e.g., organs). Our lab aims to enlist biology’s materials, mechanisms and lessons to fabricate high-performance soft matter that is cheap, safe and sustainable. In particular, we focus on building structure/function using stimuli-responsive biological polymers (especially polysaccharides), enzymes (especially tyrosinase and transglutaminase), and redox-active phenolics. Currently we are collaborating with several groups from around the world in three primary areas of research.
Biofabrication of the Bio-device Interface
The last century witnessed spectacular advances in both microelectronics and biotechnology yet there was little synergy between the two. A challenge to their integration is that biological and electronic systems are constructed using divergent fabrication paradigms. Biology fabricates bottom-up with labile components while microelectronic devices are fabricated top-down using methods that are “bio-incompatible”. Biofabrication – especially the use of biological materials and mechanisms for construction – offers the opportunity to span these fabrication paradigms by providing convergent approaches for building the bio-device interface.
Figure 1 (below) illustrates our vision for biofabricating the bio-device interface. Device-imposed stimuli provide the cues to initiate assembly and control spatiotemporal localization. Integral to this vision are stimuli-responsive materials that recognize the device-imposed cues and respond by undergoing a sol-gel transition to deposit as stable hydrogel films. Importantly, hydrogel electrodeposition provides a mechanism to trigger self-assembly over a hierarchy of length scales and yields a water-rich microenvironment that is generally compatible with labile biological systems. A second mechanism for hierarchical assembly employs enzymes that covalently conjugate macromolecules (e.g., proteins) to the self-assembled matrix. Through various collaborations, we are expanding and applying these biofabrication tools to biofunctionalize microfluidic lab-on-a-chip devices.
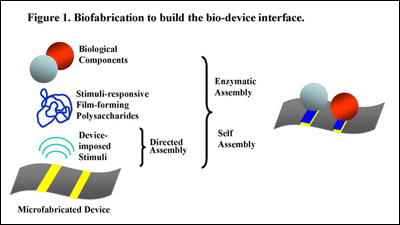
Fig. 1. Biofabrication to build the bio-device interface.
Biofabrication 2022002 (2010).
Biofabricated Redox-capacitor to Establish Bio-device "Connectivity"
Biology and electronics each possess incredible signaling processing capabilities – but they use different signaling modalities. Biology signals using ions and molecules while electronic devices use electrons to process information. Oxidation-reduction (i.e., redox) reactions provide the bridge to connect biological and electronic communication.
We recently fabricated a bio-based redox-capacitor by depositing a thin film of the polysaccharide chitosan and then modifying this film with catecholic moieties. Catechols/quinones are common redox-couples in biology and we observed that these moieties confer redox-activity to the chitosan film. Importantly, the catecholic matrix is redox-active (it can exchange electrons with appropriate mediators) but is non-conducting (it cannot exchange electrons directly with the underlying electrode). These physicochemical properties allow the catecholic matrix to be switched between two stable states (oxidized and reduced) and also allows the matrix to gate, amplify and partially-rectify mediator currents. Also, this catecholic matrix can exchange electrons with bio-relevant oxidants and reductants (e.g., NADH and ascorbate). As illustrated in Figure 2 (below), this catecholic redox-capacitor can interconnect biological and electrical inputs/outputs for information processing.
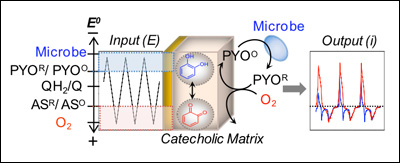
Figure 2. The catecholic matrix (QH2/Q) can exchange electrons with the redox-active metabolites pyocyanin (PYO) and acetosyringone (AS) that can access cellular redox-state. In addition, these metabolites can shuttle electrons between the electrode and the matrix. These properties allow cyclic potential inputs to be imposed to generate steady output currents that possess information of biological/environmental redox activities.
Biopolymeric Materials as High-performance Alternatives
Traditionally, biopolymeric materials have been viewed as bio-based alternatives to synthetic polymers. The commonly-stated advantages of bio-based polymers are their sustainable source and their environmental-friendliness (i.e., biodegradability). However, biopolymers often possess functionality that allows them to compete in terms of performance. In fact, the high-performance capabilities of proteins are well-established: proteins are expressed with controlled sequence, size and shape, and they possess unparalleled capabilities for molecular recognition and catalysis.
Our lab focuses on polysaccharides and phenolics. Polysaccharides are routinely used in food and consumer applications because they possess valuable functional properties (e.g., viscosifying and gelling properties). Often these properties change dramatically in response to small changes in environmental conditions (e.g., in pH, temperature and solution composition). Phenolic-based materials (e.g., lignin and melanin) are more abundant in nature than either proteins or nucleic acids, yet they are seldom studied. Often phenolics possess optical and redox properties that confer protective functions. Over the long term, our lab’s biofabrication research should promote the broader use of polysaccharides and phenolics for food, cosmetic, pharmaceutical, biotechnology and medical applications where performance and biological compatibility are essential.
Coupling Electrodeposition with Layer-by-Layer Assembly to Address Proteins within Microfluidic Channels. Advanced Materials. 23: 5817-5821 (2011).
Electroaddressing Functionalized Polysaccharides as Model Biofilms for Interrogating Cell Signaling. Advanced Functional Materials. 22: 519-528 (2012).
Electrodeposition of a Biopolymeric Hydrogel: Potential for One-step Protein Electroaddressing. Biomacromolecules. 13: 1181-1189 (2012).
Biofabrication of Stratified Biofilm Mimics for Observation and Control of Bacterial Signaling. Biomaterials. 33: 5136-5143 (2012).
Biofabricating Multi-functional Soft Matter with Enzymes and Stimuli-Responsive Materials. Advanced Functional Materials. In Press (2012).
Redox Cycling and H2O2-Generation by Fabricated Catecholic Films in the Absence of Enzymes. Biomacromolecules, 12: 880-888 (2011).
Biomimetic Fabrication of Information-rich Phenolic Chitosan Films. Soft Matter, 7: 9601-9615 (2011).
Redox Capacitor to Establish Bio-device Redox-Connectivity. Advanced Functional Materials. 22: 1409-1416 (2012).
Payne, G.F., P.B. Smith (Eds). “Renewable and Sustainable Polymers.” American Chemical Society Press. Washington, DC (2011).